1996 Science Teachers Association of Texas (STAT) Convention
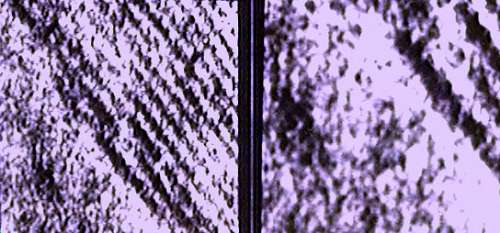
The 0.335 nm inter-atomic lattice planes of carbon graphene sheets in graphite. There are a number of different allomorphs of carbon. Graphite is the most stable therdynamically. That is, one can convert diamonds to graphite, but not graphite to diamonds. Amorphous carbon can be converted to diamons but only usually at great temperatures and pressures.
Another form of graphite is the "fullerene" or buckyballs. This is a C-60 cluster of carbon atoms, arranged in pentameric and hexameric vertices to form a closed sphere. The molecular cluster is just like a soccer ball! Professor Richard Smalley, Professor Robert F. Curl,Jr., and Sir Harold W. Kroto discovered the buckyball. This year they were awarded the Nobel Prize in Chemistry
[Previous |Next |Slide Show Table of Contents ]