Microscopy of Curdlan Structure
Iain M. Cheeseman and R. Malcolm Brown, Jr.Department of Botany, The University of Texas at Austin, Austin, Tx., 78713
Abstract:
The structure of curdlan (a (1,3)-ß-glucan) was examined using light microscopy, transmission electron microscopy (TEM), and molecular modeling. Light microscopy revealed a doughnut-shaped structure for the curdlan granule similar to that of starch. In order to study the structure of the curdlan molecule itself, a gel was prepared from the curdlan granule. Although curdlan is insoluble in neutral water, it dissolves easily in a dilute alkali solution and forms a gel on heating above 54° C. Low resolution TEM showed that the gel is composed primarily of intertwining microfibrils at lower temperatures and composed of associated microfibrils at higher temperatures. High resolution TEM determined that the microfibrils are composed of three curdlan molecules that are associated to form a triple helix, and molecular models of the triple helix were compared to these structures.
Introduction:
Curdlan is member of the class of molecules known as (1,3)-ß-glucans. These polysaccharides are characterized by repeating glucose subunits joined by a ß linkage between the first and third carbons of the glucose ring. While the primary structure (Fig. 1) is a long chain, curdlan forms more complex tertiary structures due to intramolecular and intermolecular hydrogen bonding. (1,3)-ß-glucans are involved in cell structure and food storage in bacteria, fungi and higher plants. Curdlan in particular shows strong anti-tumor properties and has utility as a food additive. A firm understanding of curdlan structure is needed in order to better understand these uses. For example, it has been shown that there is no anti-tumor activity when curdlan assumes a random-coil conformation or is composed of shorter chains, but is greatly enhanced when the curdlan is found primarily as a single helix (Saitô et al. 1990).
There is still a great deal of confusion concerning the exact structure of curdlan, because it occurs in a variety of different states. In its natural state, curdlan is poorly crystalline and is found as a granule, much like that of starch. The granule is insoluble in distilled water, but dissolves easily in a dilute alkali solution, due to the ionization of hydrogen bonds, and forms a gel when it is heated above 54° C. The gel is composed mainly of interacting microfibrils (Å100 Å in diameter) which are made up of many curdlan molecules. There are several changes that occur with regards to the gel formation due to differences in the concentration of sodium hydroxide used and the temperature at which the gel is prepared. One such change is observed as the concentration of the sodium hydroxide solution that is used to dissolve the curdlan is raised from 0.19 M to 0.22 M. While the gels made with less than 0.19 M sodium hydroxide are made of curdlan molecules with a more ordered structure, using a concentration of sodium hydroxide above 0.22 M forces the curdlan to assume a random helix conformation (Saitô et al. 1977). Additional irreversible changes occur associated with temperature. Although curdlan gelation begins at 54° C forming what is termed as a low set gel, an additional change occurs at 95° C to form what is termed a high set gel (Stone and Clarke 1992). The high set gel has the properties of being much stronger and more resilient than the low set gel. This change is explained by the hypothesis that microfibrils dissociate at 60° C as the hydrogen bonds are broken, but then reassociate at higher temperatures as hydrophobic interactions between the curdlan molecules occurs (Harada et al. 1979). An additional change to an even more ordered form is suggested in some sources (Harada et al. 1979) as the temperature is raised above 120° C.
In this study, the many levels of curdlan structure were examined. The granules and their dissolution in sodium hydroxide (caused by the disruption of hydrogen bonds) were studied using polarized light microscopy. Low magnification transmission electron microscopy (TEM) was used to examine the basic gel structure and the interaction between the microfibrils. High resolution transmission electron microscopy (HRTEM) was used to examine the fine structure of the gel to determine what composes the microfibrils and other parts of the gel, as well as the structure of the curdlan molecule. Previously it has been suggested that curdlan can exist as a triple helix, single helix, single chain, or a random coil. The results of the HRTEM study were compared with molecular models created from X-ray diffraction data of the curdlan triple helix.
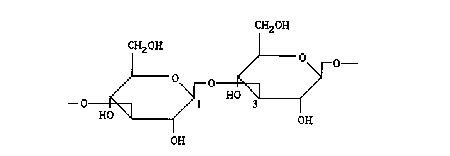
Figure 1: Primary structure of the curdlan macromolecule. A ß linkage between carbons 1 and 3 determines the formation of the tertiary structure.
Materials and Methods:
Light Microscopy:
Light microscopy was performed on a Zeiss Universal light microscope (with an objective lens magnification of 16 X) using polarizing filters and Nomarski differential interference contrast (DIC) optics. Images were captured to a Panasonic optical disk using an Optronics camera. Dry curdlan granules (courtesy, Dr. G. Franz) were placed on a glass slide and covered with a cover slip. A small amount of water was added at the edges. In order to observe the process of the curdlan dissolving, a small amount of 1.0 M sodium was added at the edges of the cover slip.
Molecular Modeling:
Atomic coordinates of the curdlan triple helix determined by X-ray crystallography were provided by Dr. A. Sarko based on a 1980 study (Deslandes et al. 1980). The coordinates were imported into ChemX™ and both atomic stick and space filling models were created.
Gel Preparations:
Between 0.020 g and 0.030 g of curdlan were dissolved in 10 ml of a varying molarity of sodium hydroxide. Concentrations of 0.1 M, 0.3 M, and 1.0 M sodium hydroxide were used. This solution was then heated at a constant temperature for 30 minutes. Gels were prepared at 65° C, 95° C, and 128° C. The gel was then dispersed by using a spatula or sonicating the solution briefly.
Electron Microscopy:
Electron microscopy was performed on a Philips 420 electron microscope using an accelerating voltage of 100 kV, a lens current of less than 5 µA, condenser and objective apertures of 100µm, and lens magnifications of between 9,600 X and 160,000 X. Specimens were prepared by dropping the gel solution onto a Formvar coated 300 mesh copper grid. They were then washed with several drops of water, several drops of 0.25% Triton-X 100, and again with several drops of water. Specimens were then negatively stained using 2% uranyl acetate (UA). A yttrium/aluminum/garnet crystal (YAG) converted the electrons into a light signal for viewing with a Gatan intensified video camera. Images were captured to an IBAS computer system and were adjusted using contrast normalization and scaling, lowpass filters, median filters, high pass filters and enlargement.
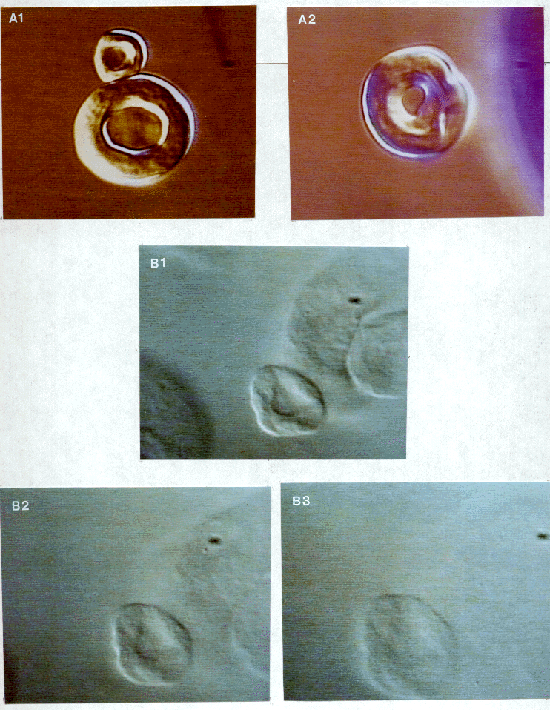
Figure 2: (A) Polarized light microscopy of the curdlan granule. The granule has a doughnut shape and occurs in two different sizes. The relatively low level of birefringence indicates that there is not a large amount of order within the granule. (B) Nomarski DIC light microscopy showing three stages of the dissolution of curdlan in 1.0 M sodium hydroxide. The total process takes about 10-15 seconds, but is probably dependent on sodium hydroxide molarity. It is possible to see both a swelling of the structure and a loss of structure until the granule finally becomes almost invisible.
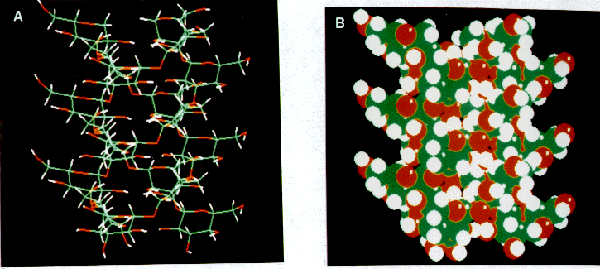
Figure 3: ChemX™ molecular models of the curdlan triple helix from X-ray atomic coordinates (coordinates courtesy of Dr. A. Sarko). (A) Stick model demonstrating the manner in which curdlan forms a triple helix. The three strands of curdlan wind tightly together to form a helix with a diameter of 15.56 Å and a fiber repeat of 18.78 Å (Chuah et al. 1983). (B) Space-filling model of (A).
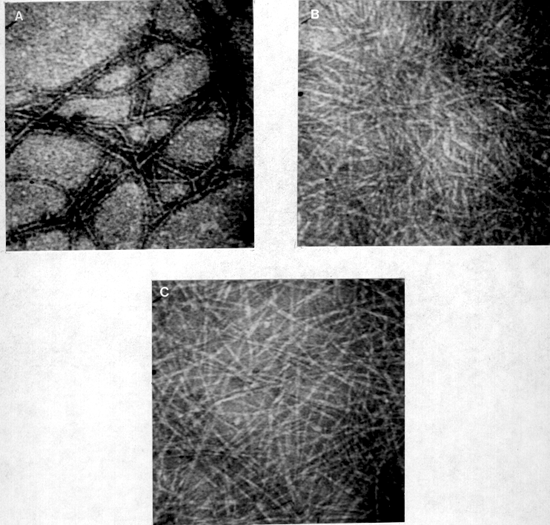
Figure 4: Low magnification images (X 100,000) of the curdlan gel structure. (A) Curdlan gel prepared at 95° C using 0.1 M sodium hydroxide. The microfibrils (100 Å in diameter) are associated to form an organized gel structure. (B) Curdlan gel prepared at 95° C using 0.3 M sodium hydroxide. The microfibrils are not associated and there is a slight swelling of the microfibrils. (C) Curdlan gel prepared at 65° C using 0.1 M sodium hydroxide. The microfibrils are not associated and appear thinner in diameter (Å50 Å).
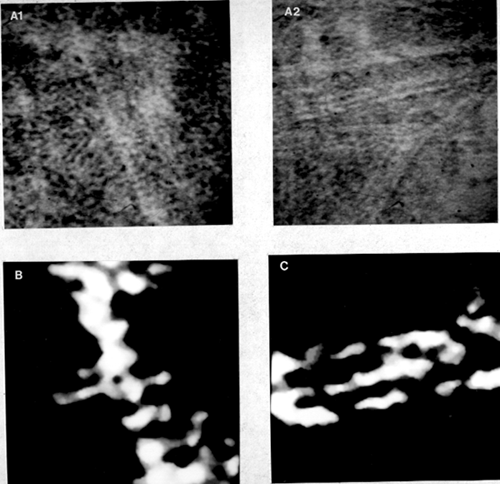
Figure 5: High resolution images of the curdlan molecule. (A) Original images (x 1,000,000). (B) Computer enhanced image of the first image in (A). Measurements indicate a diameter of about 15 Å and a periodic structure that repeats every 5.5 Å. These measurements indicate that the structure is a triple helix. (C) Computer enhanced image of the second image in (A). The curdlan molecules associate to form a microfibril by connections between molecules.
Discussion:
Granule Structure:
The low level of birefringence that was observed with polarized light (Fig. 2A) indicates that there is not a large degree of order within the granule. This suggests that the microfibrils wind around to form the doughnut shaped structure, but are not highly structured when doing so. Further knowledge is gained from the images of the curdlan dissolving in sodium hydroxide (Fig. 2B). Similar observations have been noted with cellulose (Rees 1965). In both cases this indicates that there is extensive hydrogen bonding that is holding the granule together, most likely by strongly binding the triple helices to form microfibrils and then binding together the microfibrils. When these bonds are broken the granule looses its structure. Eventually during hydrolysis, the granules can no longer be seen, indicating that the microfibrils have dissociated from each other. This was confirmed with electron microscope images of curdlan dissolved in 1.0 M sodium hydroxide (not show here). This information suggests that the curdlan granule is similar in structure to the model proposed for paramylon (another (1,3)-ß-glucan) in which the triple helices group together to form microfibrils and wind around tangentially to form the doughnut shaped structure (Marchessault and Deslandes 1979).
Basic Gel Structure:
Low magnification electron microscopy (Fig. 4) demonstrated differences in gel structure due to different methods of preparation that were consistent to those described in several sources (Koreeda et al. 1974; Harada et al. 1979). Associations between microfibrils in the gel prepared at 95° C (Fig. 4A) support the hypothesis that hydrophobic interactions occur between curdlan molecules as the temperature is increased (Harada et al. 1979) since this association was not observed in the 65° C gel (Fig. 4B). In fact, even the bonds within the microfibrils (between curdlan triple helices) are not as strong and the triple helices can be resolved even at lower magnifications. The reported transition as the sodium hydroxide concentration is raised form 0.19 M to 0.22 M (Saitô et al. 1977) was also observed. In a gel prepared with 0.3 M sodium hydroxide, the swelling of the fibers indicates an increased ability of the sodium hydroxide to break hydrogen bonds compared to the 0.1 M sodium hydroxide gel. There were, however, no differences noted in the structure of the curdlan molecule itself contrary to previous reports (Saitô et al. 1977).
Gel Ultrastructure:
High resolution electron microscopy (Fig. 5) revealed interesting insights into the conformation of the curdlan molecules and the manner in which they associate. Measurements and the observation of a periodic structure confirmed the fact that the curdlan molecule exists in the form of a triple helix. It is possible to view the similarity between the molecular model of the triple helix (Fig. 3B) and the images obtained with the high resolution TEM (Fig. 5). Similar results were noted for all gel preparations which indicates that the triple helix is present in all gel preparations. It is possible that other forms of curdlan exist, however the triple helix seems to be the major form involved in the structure of the gel. Images that show several triple helices (Fig. 5B and images not shown here) show that there is also some sort of connection between the helices. The fact that these connections exist at random points indicates that these junctions are probably formed by the unwinding of the triple helix or by associations from other curdlan molecules that are not in a triple helical form.
Conclusions:
1) The curdlan granule has a doughnut shape formed by microfibrils of curdlan winding around tangentially.
2) Curdlan gels made at higher temperatures show a larger association of triple helices and microfibrils, while gels made at higher concentrations of sodium hydroxide show a lower degree of order.
3) The main structure of curdlan for all gel states is the triple helix, though other states exist in the gel.
References:
Bluhm, T. L., Deslandes, Y., Marchessault, R. H., Pérez, S. and Rinaudo, M. (1982). “Solid-State and Solution Conformation of Scleroglucan.” Carbohydrate Research 100: 117-130.
Chuah, C. T., Sarko, A., Deslandes, Y. and Marchessault, R. H. (1983). “Triple-Helical Crystalline Structure of Curdlan and Paramylon.” Macromolecules 16: 1375-1382.
Deslandes, Y., Marchessault, R. H. and Sarko, A. (1980). “Triple-Helical Structure of (1,3)-ß-D-Glucan.” Macromolecules 13: 1466-1471.
Fulton, W. S. and Atkins, E. D. T. (1980). The Gelling Mechanism and Relatioship to Molecular Structure of Microbila Polysaccharide Curdlan. Fibre Diffraction Methods. Washington, D. C., American Chemical Society. 385-410.
Harada, T., Koreeda, A., Sato, S. and Kasai, N. (1979). “Electron Microscopic Study on the Ultrastructure of Curdlan Gel: Assembly and Dissociation of Fibrils by Heating.” Journal of Electron Microscopy 28(3): 147-153.
Kasai, N. and Harada, T. (1980). Ultrastructure of Curdlan. Fibre Diffraction Methods. Washington, D. C., American Chemical Society. 363-383.
Koreeda, A., Harada, T., Ogawa, K., Sato, S. and Kasai, N. (1974). “Study of the ultrastructure of gel-forming (1,3)-ß-D-glucan (curdlan-type polysaccharide) by electron microscopy.” Carbohydrate Research 33: 396- 399.
Marchessault, R. H., Deslandes, Y., Ogawa, K. and Sundararajan, P. R. (1977). “X-Ray diffraction data for ß- (1,3)-D-glucan.” Canadian Journal of Chemistry 55: 300-303.
Marchessault, R. H. and Deslandes, Y. (1979). “Fine Structure of (1,3)-ß-D-Glucans: Curdlan and Paramylon.” Carbohydrate Research 75: 231-242.
Rees, D. A. (1965). “Structure, conformation, and mechanism in the formation of polysaccharide gels and networks.” Journal of Clifford, Trans. Farradays 61: 267-332.
Saitô, H., Ohki, T. and Sasaki, T. (1977). “A 13C Nuclear Magnetic Resonance Study of Gel-Forming (1,3)-ß- D-Glucans. Evidence of the Presence of Single-Helical Conformation in a Resilient Gel of a Curdlan- Type Polysaccharide 13140 from Alcaligenes faealis var. myxogenes IFO 13140.” Biochemistry 16(5): 908-914.
Saitô, H., Yoshioka, Y., Yokoi, M. and Yamada, J. (1990). “Distinct Gelation Mechanism between Linear and Branched (1,3)-ß-D-Glucans as Revealed by High-Resolution Solid-State 13C-NMR.” Biopolymers 29: 1689-1698.
Stone, B. A. and Clark, A. E. (1992). Chemistry and Biology of (1,3)-ß-Glucans. Victoria, Austrailia, La Trobe University Press.
Acknowledgments:
We wish to thank Dr. G. Franz for providing the curdlan used in this study, Dr. A. Sarko for providing the atomic coordinates that were used to model the curdlan triple helix, and Judith A. Sharp for her advice and help with electron microscope techniques. This work was funded in part by a grant from Howard Hughes through the Summer Undergraduate Research Program in Molecular Biology at the University of Texas at Austin.