A COMPARATIVE STUDY OF IN VITRO CELLULOSE SYNTHESIS AMONG SEVERAL HIGHER PLANTS
Krystyna Kudlicka, Jong H. Lee, and R. Malcolm Brown, Jr.Department of Botany, The University of Texas at Austin, Austin, Tx., 78713
ABSTRACT
A comparative study was undertaken of cellulose synthesized in vitro from corresponding cellular tissues active in primary and secondary wall synthesis. Etiolated mung bean seedlings active in primary wall synthesis and fibers of cotton active in secondary wall synthesis were chosen for comparison. Identical enzyme extraction protocols were used for both systems. Mung bean fractions (PM and SE2) incorporated more radioactive UDP-Glc into the in vitro product than did the same fractions from cotton. A significant difference was found with the mild digitonin-extracted fraction of mung bean (SE1) which produced eight times more total product than did the SE1 fraction of cotton. The cotton SE1 enzyme fraction produced the largest quantity of cellulose (32.1% of total glucan activity) in comparison with mung bean (6.9%). In addition, AN reagent treatments for different periods, suggest that the cellulose synthesized in vitro from mung bean is more easily degraded than cellulose synthesized in vitro from cotton fibers. This would imply that cellulose I produced in vitro from the cotton SE1 fraction may have a higher crystallinity and DP than the cellulose I produced from mung bean. These findings are indirectly supported by TEM analysis of the fibrils of cellulose I produced by the SE1 fraction of mung bean. In this case, the fibrils are loosely associated in comparison with the compact bundles of cellulose I synthesized by the cotton SE1 fraction. In both systems, the SE2 fractions produced only cellulose II and abundant ß-1,3 glucans.
INTRODUCTION
All higher plants contain ß-glucan synthases, which are responsible for the biosynthesis of ß-1,4-glucans (cellulose); however, they also synthesize ß-1,3-glucans (callose) in response to wound, physiological stress, or infection. The nature of the enzymes involved in the synthesis of these important polysaccharides and the control of their activities during wall formation are not well understood.
Considerable efforts have been made to achieve in vitro synthesis of cellulose using membrane preparations of various degrees of purity from different organisms (Carpita, 1982; Maclachlan, 1982; Blaschek et al., 1983; Delmer, 1987, 1991; Fink, Jeblik, and Kauss, 1990; Dhugga, and Ray, 1991; Read, and Delmer, 1991, Li, and Brown, 1993; Okuda et al., 1993). Almost all attempts at in vitro production of cellulose have resulted either in the formation of ß-1,3-glucans or only very limited synthesis of ß-1,4-glucans, in the latter case, without sufficient proof of the characteristic crystallinity of the product (Franz and Blaschek, 1990).
By modification of the extraction and solubilization procedures, we have recently increased the quantity of in vitro cellulose to 32.1% of the total glucan product. This cellulose gave a diffraction pattern typical of the native cellulose I allomorph (Kudlicka et al., 1995).
In the present investigation, we have compared in vitro cellulose products synthesized from membrane preparations isolated from secondary walls of cotton fibers and primary walls of etiolated mung bean seedlings. We have applied the same conditions found to be essential for in vitro cellulose I assembly from cotton (Kudlicka et al., 1995). We have also employed native gel electrophoresis (Dhugga and Ray, 1994) to test activities of separated fractions and to examine these fractions for the type of product made.
MATERIALS AND METHODS
The cotton balls were harvested 20 days after anthesis, and the locules were removed and stored in liquid nitrogen as described previously Okuda et al., 1993, Kudlicka et al., 1995). Mung bean seedlings were grown in the dark at 28-300C in vermiculite saturated with water and harvested after 3-5 days. In addition actively growing Arabidopsis plant were used for the extraction of proteins involved in in vitro glucan synthesis.
Plasma membrane fractions of cotton and mung bean were prepared in 50 mM Mops buffer with 0.25 M sucrose according to Kudlicka et al., (1995). The solubilized enzyme fractions of cotton and mung bean were obtained through a separate two-step digitonin solubilization. The SE1 fraction was solubilized in 0.05% digitonin, and then the SE2 fraction was solubilized in 1.0% digitonin. Mung bean and Arabidopsis membrane fractions also were solubilized in increasing concentrations of digitonin (from 0.05 to 1.0%) and fractionated under native gel electrophoresis.
The activities of different enzyme fractions were assayed using 14C-UDP-glucose. The in vitro products were synthesized using cold UDP-glucose and examined with transmission electron microscopy. Native gel electrophoresis was performed according to Dhugga and Ray (1994) with certain modifications.
RESULTS AND DISCUSSION
A comparison of the glucan synthase specific activities of PM, SE1, and SE2 isolated from mung bean and cotton shows that all mung bean fractions incorporated more radioactive UDPG into the total product than fractions isolated from cotton (Table 1, parts A and B). The percentage of cellulose in different fractions of mung bean and cotton vs. total glucan revealed that all fractions of cotton produced more cellulose than mung bean fractions (Table 1, part C).
The SE1 and SE2 fractions produced abundant fibrillar material (Fig. 1A, B), which is very similar to the product from cotton enzyme fractions characterized earlier as ß-1,3-glucan (Okuda et al., 1993; Kudlicka et al., 1995). Both fractions also synthesized materials strongly labeled with the CBH I-gold complex; however, these materials had a different morphology (Fig. 1 A, B). The material synthesized by the SE2 fraction (Fig. 1 A) is identical to cellulose II described earlier from cotton (Okuda et al., 1993; Kudlicka et al., 1995).
The SE1 product shows a larger quantity of labeled, fibrillar material with elongated microfibrils arranged into a loose bundle dispersed in between ß-1,3-glucans (Fig. 1 B, 2 A). The electron diffraction pattern of the microfibrils is shown in Fig. 3 D with reflections characteristic for cellulose I. Cellulose I synthesized by the SE1 fraction from cotton fibers shown in Fig. 2 B, with microfibrils very tightly associated and arranged into a compact bundle, much as they occur in the intact secondary cell wall.
In vitro synthesis of two morphological forms of cellulose I by two different organisms suggests different mechanisms of cellulose I crystallization. This relates to the density and arrangement of the catalytic sites within the enzyme complex. If these sites are spatially distorted or further apart, the crystallinity of the cellulose I assembled is predicted to be less ordered. Perhaps mung bean cellulose synthases may be specific for a given developmental stage within the plant, or different among various plant species (e.g., mung bean vs. cotton or primary vs. secondary wall stages of development).
The greater resistance to AN of the in vitro cellulose synthesized by cotton suggests the possibility of a higher molecular weight product. If so, this would parallel the distribution of in vitro synthesis of cellulose in cotton secondary walls. This raises the interesting question that one or more different cellulose synthases may be operative at various developmental stages of the life cycle in a given organisms.
It is interesting that only the SE1 fraction produces cellulose I, whereas the SE2 fraction produces cellulose II in both cotton and mung bean systems. A double digitonin solubilization is an effective process in cotton; however, these same conditions are less effective for solubilization of cellulose synthases from mung bean (Table 1, part B). Preliminary results of the gradual solubilization of membrane proteins with increased concentrations of digitonin show that the most effective solubilization was achieved with 0.25% digitonin (122.03nmol/min/mg protein) and followed 0.5% (113.02nmol/min/mg).
Native gel electrophoresis of the above fractions followed by incubation with UDPG, produced a single band of polymeric product at the top of the gel. This band was detected by fluorescence under UV light, after treating the gel with Tinopal (Fig. 5 A). The single band of glucan activity in the native gel corresponds to a single Coomassie-blue-stained protein band (Fig. 5 B). After removing the acrylamide from the band synthesized at the top of gel, and observing the product in TEM, it was evident that it comprise three different structures corresponding to callose (a), cellulose I (b) and cellulose II (c) (Fig. 5 C).
When a separate 3% stacking/6% running gel was used for separation of the most active fractions under nondenaturating conditions, two bands appeared after incubation with UDPG: (a) a sharp band at the top of the stacking gel; and, (b) a broad band at the top of the running gel Fig. 6 A). The product from the stacking gel was exclusively fibrillar material strongly labeled with CBH I-gold complex indicating only cellulose (Fig. 6 C). Interestingly, the band from the running gel revealed only callose (Fig. 6 D). This appears to be the first result of an exclusive separation of ß-1,4- and ß-1,3-glucan synthesis activity in vitro. It will be interesting to compare the proteins from these two bands (work in progress).
Similar separations were obtained from Arabidopsis solubilized fractions; however, in the running gel, the active band migrated further into the gel (Fig 7 A, B). The Arabidopsis results are continuing; however, it is interesting to note that the most prominent native band when separated by SDS, revealed only a 54 kD polypeptide (Fig. 7 C). This could be callose synthase, similar to that identified by Dhugga and Ray (1994) from pea. If the product synthesized by this band is found to be only callose, these suggestions would be greatly strengthened.
This research was supported, in part by grants from DOE GDE-FG03-94ER20145; USDA 93-37301-9303; and Welch F-1217.
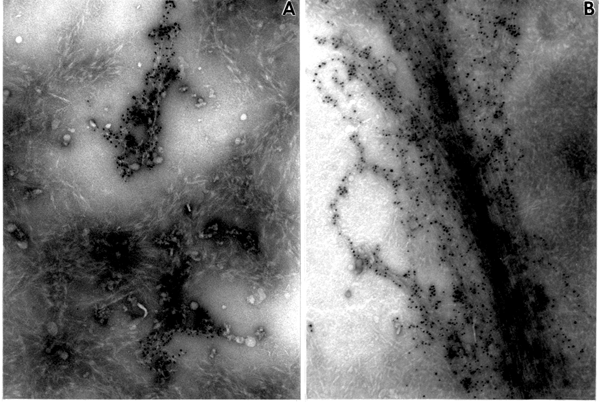
Fig. 1. In vitro product synthesized by mung bean enzyme fractions. Note the difference in morphological appearance of products in A and B. (A). Product synthesized by the SE2 fraction. Abundant fibrous material not labeled with CBH I-gold complex, indicating ß-1,3-glucans, and small disorganized aggregates indicating cellulose II with CBH I-gold particles attached, x90,000. (B) Product synthesized by the SE1 fraction. Long fibrils of cellulose I strongly labeled with CBH I-gold complex embedded in ß-1,3-glucan, x90,000.
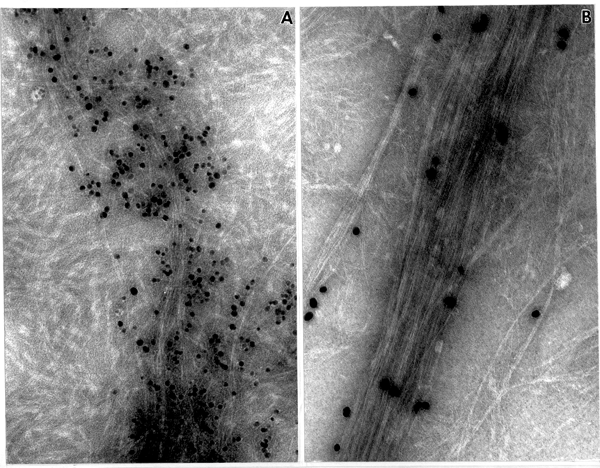
Fig. 2. ß-glucan products synthesized in vitro by SE1 fractions. (A) Mung bean SE1 fraction. Note loose bundle of fibrils strongly labeled with CBH I-gold complex (=cellulose I), x205,000. (B). Cotton SE1 fraction. Long fibrils labeled with CBH I-gold complex (=cellulose I) are arranged into a compact bundle, surrounded by ß-1,3-glucan, x205,000.
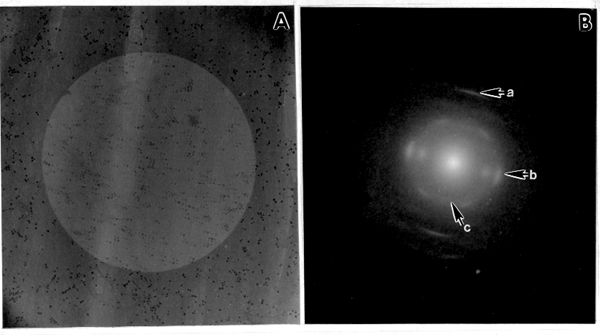
Fig. 3. ED pattern of in vitro product from mung bean. (A) Defocused image of the in vitro product synthesized by the SE1 fraction. The product is highly oriented from top to the bottom of the electron micrograph. (B) ED pattern of the same field showing a prominent meridianal 004 (a) and three equatorial reflections, 200, 110, and 110 (b), characteristic for cellulose I. The 002 (c) reflection is characteristic for the cellulose I ß suballomorph.
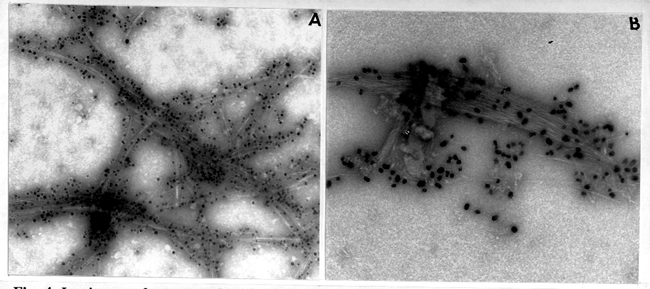
Fig. 4. In vitro product treated with AN reagent. (A) Product synthesized by mung bean SE1 fraction treated 20 min with AN. Note thin fragmented bundles of cellulose microfibrils, x150,000. (B) In vitro product synthesized from cotton SE1 fraction after a 30 min treatment with AN. The cellulose microfibrils are arranged into a compact bundle, much like that found in vivo during secondary wall synthesis, x150,000.
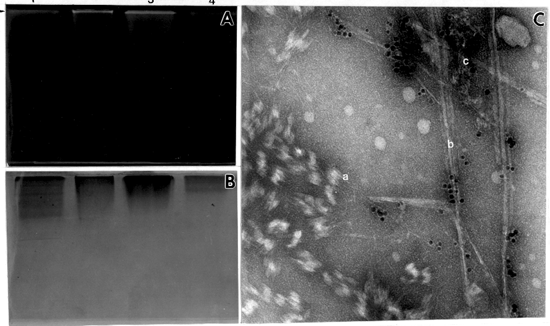
Fig. 5. Native gel electophoresis of mung bean solubilized fractions incubated with UDP-glucose for 12 hr. (A) Localized in vitro product (arrow) visualized under UV after staining with Tinopal, lane 1-fraction solubilized in 0.1% digitonin, lane 2-fraction solubilized in 0.25% digitonin, lane 3-fraction solubilized in 0.5% dogitonin, lane 4-fraction solubilized in 1% digitonin. (B) The same gel stained with Coomassie blue. (C) Negatively stained product synthesized in the gel, after removing the polyacrylamide. (a) callose, (b) cellulose I, (c) cellulose II, x 150,000.
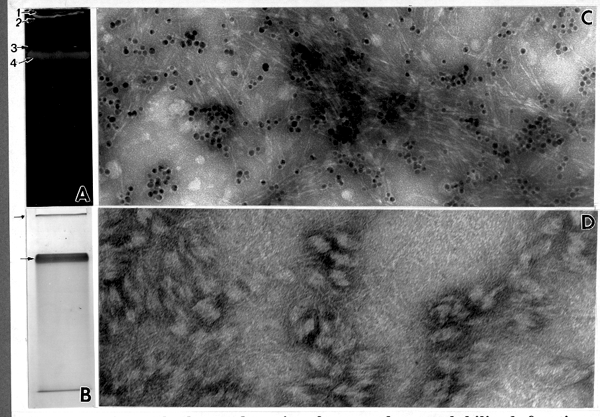
Fig. 6. Native gel electrophoresis of mung bean solubilized fraction, separated on two phase stacking and running gel. (A) Two product bands detected under UV after staining the gel with Tinopal. 1-loading well, 2-in vitro product in the stacking gel, 3-running gel boundary, 4-in vitro product localized in running gel. (B) Three protein bands in the same gel stained with Coomassie blue. Note that two of these bands (arrow) co-localize with the in vitro product. (C) The product released from the top of the stacking gel. Note CBH I-gold labeling indicating almost pure cellulose, x205,000. (D) The product released from the top of the running gel (stained with CBH I-gold). Note by the absence of labeling, the presence of only ß-1,3-glucan, x337,500.
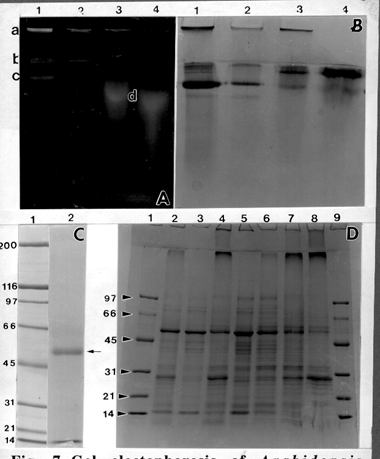
Fig. 7. Gel electophoresis of Arabidopsis fractions. (A) Product bands in the native gel visualized under UV and Tinopal staining. Lane 1-fraction solubilized in 0.1% digitonin, lane 2-fraction solubilized in 0.25% digitonin, lane 3-fraction solubilized in 0.5% digitonin, lane 4-fraction solubilized in 1% digitonin. a-stacking gel, b-running gel boundary, c-product in running gel, d-spread product in running gel. (B) The same gel stained with Coomassie blue. (C) SDS gel, (lane 1) molecular weight marker, (lane 2) 54 kD band (arrow) eluted from broad band from lane 1 of B. (D) Gradient SDS gel of different Arabidopsis fractions. Lanes 1 and 9-molecular weight marker; lane 2-crude fraction; lane 3-supernatant fraction; lane 4 membrane fraction; lane 5-fraction solubilized in 0.1% digitonin (same as lanes 1 in Figs. A and B); lane 6-fraction solubilized in 0.25% digitonin; lane 7-fraction solubilized in 0.5% digitonin; lane 8-fraction solubilized in 1% digitonin.
| A. Cotton | Fraction | Total protein (mg) | Glucan synthase activity | Cellulose synthase activity | % of cellulose synthase activity vs. total glucan synthase activity | ||
| Total activity (units) | Specific activity (units/mg) | Total activity (units) | Specific activity (units/mg) | ||||
| PM | 33.6 | 760.5 | 22.71.9 | 50.6 | 1.50.3 | 6.61.2 | |
| SE1 | 1.4 | 8.0 | 5.71.2 | 2.5 | 1.80.3 | 32.12.3 | |
| SE2 | 3.5 | 52.8 | 15.64.6 | 4.3 | 1.30.3 | 8.62.6 | |
| B. Mung bean |
PM | 69.7 | 4801.7 | 69.07.1 | 110.9 | 1.60.1 | 2.40.6 |
| SE1 | 15.9 | 743.2 | 46.65,6 | 47.2 | 2.90.3 | 6.90.7 | |
| SE2 | 17.5 | 977.9 | 56.16.3 | 20.7 | 1.201 | 2.10.5 | |
| C. | Fraction | Comparison of glucan synthase specific acticvity of mung bean and cotton | Comparisom of cellulose synthase specific activity of mung bean and cotton | Comparison of % of cellulose synthase activity vs. total glucan synthase activity of mung bean and cotton |
| PM | 3.0 x > | = | 2.8 x < | |
| SE1 | 8.2 x > | 1.6 x > | 4.7 x < | |
| SE2 | 3.6 x > | = | 4.1 x < |
| Fraction | Glucan synthase specific activity | 10min treatment with AN | 20min treatment with AN | 30min treatment with AN | |||
| (U) | (%) | (U) | (%) | (U) | (%) | ||
| CF | 21.53 | - | - | - | - | 1.54 | 7.15 |
| PM | 58.34 | - | - | - | - | 2.00 | 3.43 |
| SE1 | 45.42 | 5.48 | 12.07 | 4.66 | 10.26 | 3.22 | 7.09 |
| SE2 | 55.74 | 3.39 | 6.08 | 2.87 | 5.15 | 1.90 | 3.41 |
REFERENCES
Blaschek, W., D. Haass, H. Koehler, U. Semler, and G. Franz. 1983. Zeitschrift fur Pflanzenphysiologie 111: 357-364.
Carpita , N. C. 1982. In R. M. Brown Jr. [ed], Cellulose and other natural polymer systems. biogenesis, structure, and degradation, 225-242. Plenum Press.
Delmer, D. P. 1987. Annual Review of Plant Physiology 38: 259-290.
Demer, D. P. 1991. In C. Lloyd [ed.], The Cytoskeletal Basis of Plant Growth and Form, 101-107. Academic Press.
Dhugga, K. S., and P. M. Ray. 1991. FEBS Letters. 278: 283-286.
Dhugga, K. S., and P. M. Ray. 1994. European Journal of Biochemistry 220: 943-953.
Fink, J., W. Jeblick, and H. Kauss. 1990. Planta 181: 343-348.
Franz, G., and W. Blaschek. 1990. In P. M. Dey, and J. B. Harborne [eds.], Methods in plant biochemistry, Vol. 2, Carbohydrates, 291-322. Academic Press, London.
Kudlicka, K., R. M. Brown Jr., L. Li, J. H. Lee, H. Shin, and S. Kuga. 1995. Plant Physiology 107: 111-123.
Li, L., and R. M. Brown Jr. 1993. Plant Physiology 101: 1143-1148.
Maclachlan, G. (1982) In R. M. Brown Jr. [ed.], Cellulose and other natural polymer systems, 227-339. Plenum Press.
Okuda, K., L. Li, K. Kudlicka, and R. M. Brown Jr. 1993. Plant Physiology 101: 1131-1142.
Read, S. M., M. Thelen, D. P. Delmer. 1991. In C. Haigler, and P. Weimer [eds.], Biosynthesis and biodegradation of cellulose, 177-200. Marcel Dekker.